News
Baoshilai is a high-tech enterprise located in China, focusing on research and development (R&D) of advanced material engineering and manufacturing key components of equipment for hydrogen energy. It has established an industrial base integrating a R&D center and supply chain cluster. As a state-level small and medium-sized science and technology enterprise, a private Jiangsu enterprise, and a Suzhou unicorn cultivation enterprise, Baoshilai has established a Changshu Enterprise Innovation Center and a Suzhou Research Center of Electrode Engineering Technology.
Baoshilai breaks through the technical barriers and has made remarkable achievements through its enthusiasm and unremitting efforts in R&D and market development. It has successfully developed a series of innovative electrodes, including BSL-1.0, BSL-4.0, and BSL-5.0 electrodes and the newly launched BSL-7.0, BSL-W, and BSL-L electrodes. These products demonstrate excellent performance in electrode stability, catalytic activity, resistance to fluctuating load operation attenuation, and other aspects.. Among them, BSL-1.0 is the only kind of domestic hydrogen electrodes certified by DEKRA, it not only enhances the brand image, but also demonstrates the stability of its product quality and its alignment with international standards. The following passage will introduce the R&D journey of Baoshilai to uncover innovation behind it.
1.Challenges of Commercial Electrodes for Alkaline Electrolysis
The operating conditions of commercial alkaline electrolyzers are usually high temperature (60-95°C), high pressure (~1.6 MPa), high-concentration alkaline environment (30 wt% KOH), and continuous flushing by circulating electrolyte. Therefore, stringent requirements are imposed on the parts applied in electrolyzers. As the active center for the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in water electrolysis, electrodes not only need to withstand the working conditions but also require excellent catalytic activity and stability.
1.1 Catalytic Activity of Electrodes
The catalytic activity of electrodes is one of the most important factors for the energy consumption of electrolyzers. From a microscopic perspective, the main factors affecting electrode activity include the intrinsic activity of active sites (that is, the turnover frequency (TOF) of unit active site) and the number of active sites (the active specific surface area SSA) of electrodes. The higher the intrinsic activity and the larger the SSA of the electrode, the better its catalytic activity is. The development direction of commercial electrodes is to introduce a porous catalytic coating on the surface of the Ni substrate, thereby increasing the active SSA and mass diffusion channels. This strategy is usually more effective on the cathode side. It is because on the one hand, the cathodic hydrogen evolution overpotential in alkaline water is relatively high, and increasing the specific surface area can significantly reduce the hydrogen evolution overpotential. On the other hand, after the long-term oxidation of the anode, its high SSA coating constructed by surface engineering will gradually be flattened and passivated because of the point effect, and the performance improvement effect will be greatly reduced.
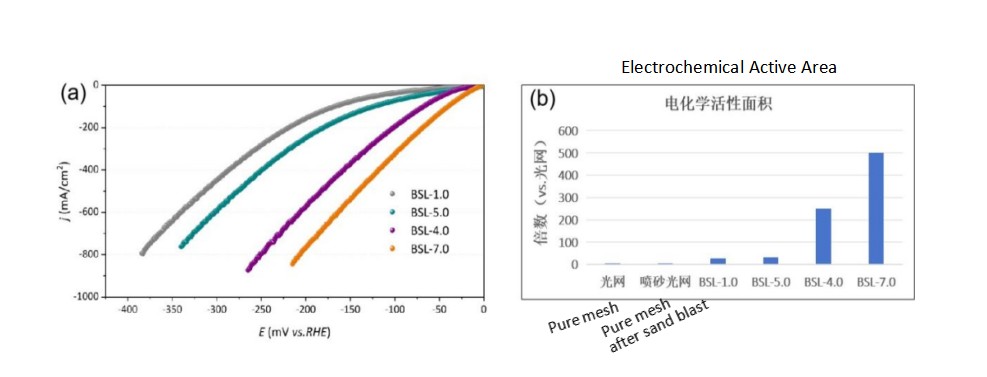
Fig.1 (a) Catalytic activity of electrodes, (b) Electrochemical specific surface area of the electrodes. (testing conditions: 30 wt% KOH at 80℃, the series internal resistance is 0.13-0.15 Ω, the electrochemically active area is calculated by double-layer capacitance.)
The typical catalytic coating is Raney nickel. Through the dealloying of Ni-Al, Ni-Zn, and other alloys, porous nickel coating is formed. With different alloy precursors, such as pseudo-alloys, solid solutions, intermetallic compounds, etc., catalytic coatings with different pore sizes and SSAs can be formed.
As for pseudo-alloys, because of the existence of micron-sized metal Al phase inside the alloy, the dealloying is mainly the direct reaction of the sacrificial phase metal with the activation liquid, and the diffusion of soluble substances in porous, the dealloying rate is fast and more efficient, and the aluminum residue is usually less than 3%. The larger Al phase also results in the formation of micropores, and the increased SSA is usually an order of magnitude higher compared with that of the nickel substrate.
For precusor alloys are solid solutions or intermetallic compounds, nickel atoms will undergo nucleation and re-growth due to the dissolution of sacrificial metal during activation. The formed metallic nickel phase usually has a mesoporous structure. The improvement of SSA is usually more than 2 orders of magnitude and the performance can even be comparable to that of noble metal electrodes. Since the dissolution of sacrificial atoms is completed through lattice diffusion, the dissolution rate is slow, and the residual of sacrificial atoms is high. For example, if Al is the sacrificial atom, the residual of Al in the nickel coating is usually greater than 10%. High Al residue can also lead to a series of adverse consequences, such as the occlusion of the electrode active site, diaphragm plugging, impurity aggregation, electrolyte pollution, and so on.
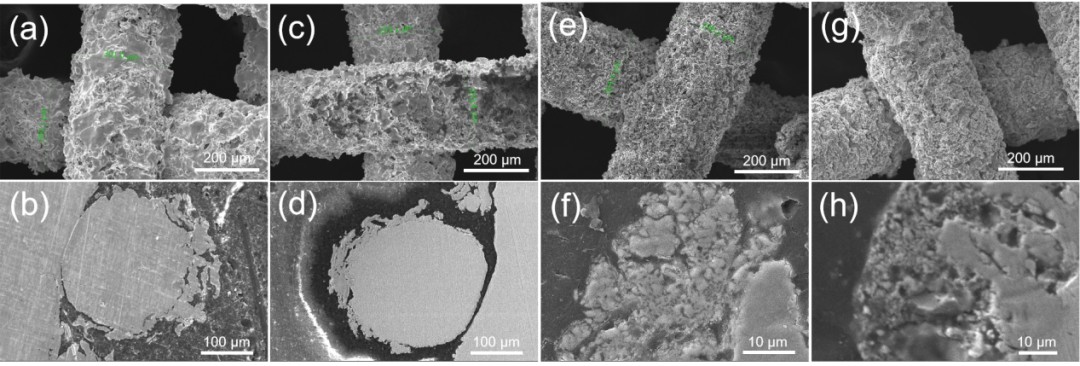
Fig.2 SEM images of surface and cross sections of electrodes with different SSAs: (a-b) BSL-1.0, (c-d) BSL-5.0, (e-f) BSL-4.0, (g-h) BSL-7.0.
1.2 Stability of the Electrodes
Another key index of electrode is durability. Durability is mainly reflected in two aspects: the stability of electrode structure and catalytic performance. For commercial electrodes, stable performance is the guarantee of the operating energy consumption, and it is mainly determined by the stability of active ingredients and coating structure.
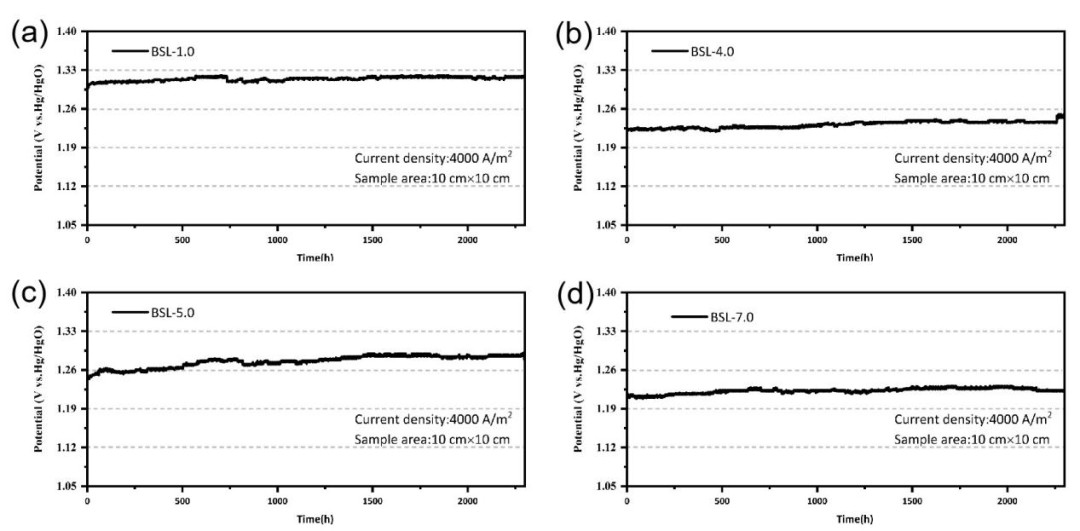
Fig. 3 Constant current decay curves of electrodes with different SSAs (test conditions: the constant current density is 4000 A/m², the tested electrode area is 100 cm², the electrolyte is 30wt% KOH, the temperature is 80⁰C, at atmospheric pressure, the reference electrode is Hg/HgO with salt bridge, and calibrated twice every day).
The stability of active sites is related to the properties of materials. Active materials should have a low degradation rate under operating conditions to meet the requirements of commercial electrodes for 20 years.
The stability of the structure is not only related to performance but also to the operation safety of electrolyzers. At present, most catalytic coatings (microporous structure coatings) are on the surface of the nickel mesh substrate by processes like thermal spraying. The bonding strength between the coating and the substrate is the key to structural stability. During catalysis, disturbances such as the liquid flow and bubbles continuously impact the coating. Insufficient bonding strength can easily cause the coating to fall off and the loss of active materials. For electrolyzers, the shedding of the coating is not only an increase in energy consumption but also deposition in the diaphragm and flow channel, causing safety risks such as short circuit.
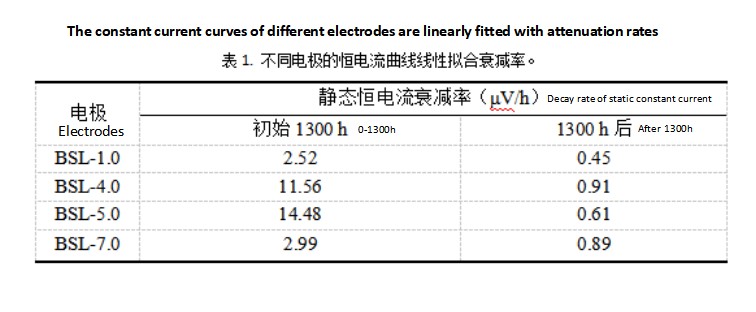
The stability of electrode activity can be characterized by the constant current mode. The BSL-1.0, BSL-4.0, BSL-5.0, and BSL-7.0 electrodes are all featured with Raney nickel coatings. The bonding strength between the coating and the substrate is relatively high. In the constant current mode (static without forced circulation of alkaline solution), they all show excellent catalytic stability. The attenuation rate after 1300 hours is lower than 1 μV/h.
1.3 Electrode Start-stop Resistance
Actual start-stop usually have a significant impact on the electrolyzers. The more series-connected cells in the electrolyzers, the larger the electrode diameter and the higher the electrode specific surface area, the more pronounced the impact. The main effect stems from reverse current during start-stop. The impact of reverse current is mainly manifested on the cathode coating. When reverse current strikes, the coating material oxidizes, greatly accelerating the oxidation and corrosion of nickel-based materials, leading to rapid corrosion, shedding, and deactivation of the coating.
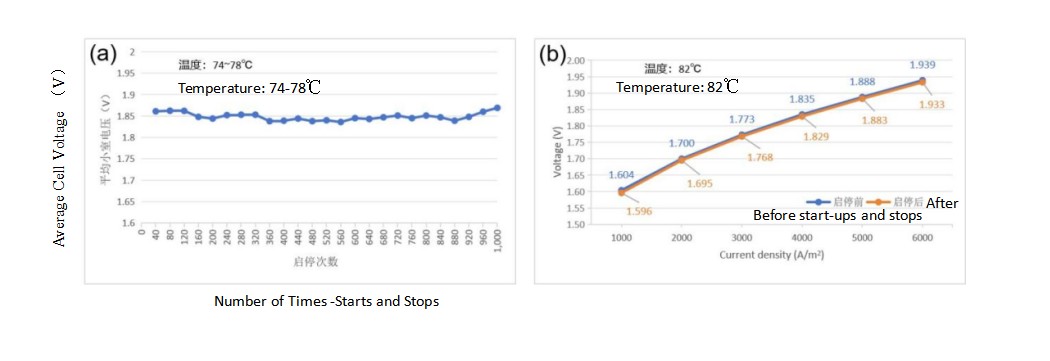
Fig. 4 (a) Changes in the average cell voltage of the BSL-5.0 electrode during 1000 start-stop in the electrolyzers, (b) The average cell voltage of BSL-5.0 before and after start-stop.
In the actual electrode development and evaluation, using forward current to impact the cathode material to simulate the impact of reverse current during the start-stop of the electrolyzers can, to a certain extent, laterally compare the start-stop resistance performance of different electrodes. Generally, electrodes whose performance and coating structure can be stably maintained after forward current impact are more resistant to the start-stop of the electrolyzers.
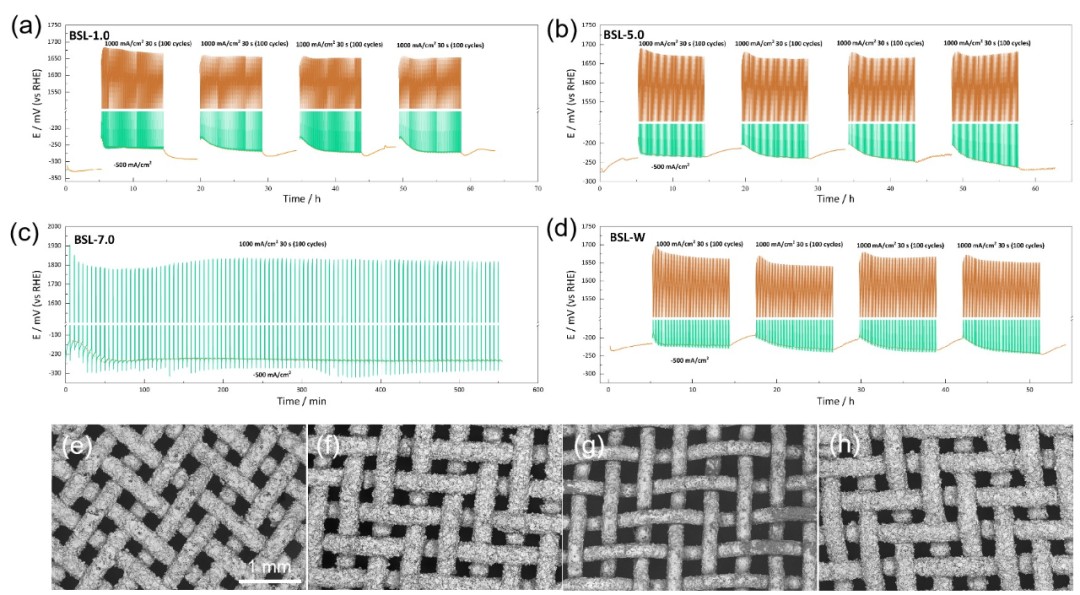
Fig. 5 (a-d) Stability of the electrodes under the impact of +10000 A/m² reverse current (test conditions: after it is operated stably at a constant current of-5000 A/m², a +10000 A/m² current density is applied for 30 seconds, then it is operated at-5000 A/m² constant current for 5 minutes. One reverse current impact is one cycle, and 100 cycles are one period), (e-f) The surface SEM images of BSL-1.0, 5.0, 7.0 and W series after one period of reverse current impact.
1.4 Contradictions in Electrode Development
The mainstream technical route of commercial alkaline water electrodes is to improve the catalytic activity of coating by increasing the SSA of nickel coatings. Baoshilai has updated its Raney nickel electrodes from BSL-1.0, BSL-5.0, BSL-4.0 to BSL-7.0. Their electrochemical active areas are about 28 times, about 32 times, about 251 times, and 500 times that of plain mesh. Among them, BSL-1.0 and BSL-5.0 show excellent stability in commercial alkaline cells. Both BSL-4.0 and BSL-7.0 have high SSA and excellent catalytic activity, BSL-7.0 is stable and its performance can even be compared with the performance of noble metal electrodes. However, there are performance attenuation and other problems in conventional alkaline electrolyzers during long-term start-stop operation.
The contradiction between the activity and stability brought by high specific surface area electrodes is mainly because of the harsh operating environment of alkaline tanks. Microscopically, the pore walls among the pores of specific surface area electrodes are usually thinner, with a thickness ranging from a few nanometers to several hundred nanometers. The liquid flow and bubbles have a more conspicuous impact on high specific surface area nickel electrodes. During physical disturbances, the thin walls are more liable to collapse, causing the active substances to fall off. For electrodes with an extremely high specific surface area, the partial loss of the coating doesn’t fully affect the electrode’s activity. This is mainly because the overly high specific surface area limits the material diffusion during the reaction. However, the loss of the coating will affect the safe operation of the electrolyzers.
Nickel electrodes with high specific surface area are usually formed through the lattice diffusion dissolution of sacrificial atoms and the nucleation and re-growth of nickel atoms. During activation, the coating has a large volume shrinkage and a high degree of looseness, and the activation is incomplete. During operation, local block-like shedding of the coating is likely to occur. Therefore, the shedding of electrodes with high specific areas often does not start at the interface between the coating and the substrate.
Nickel with a high specific surface area is more prone to oxidation, especially in the process of resisting reverse current impact, and it is difficult to recover under the reducing current, which easily leads to the overall deactivation of the surface coating. In addition, electrodes with a high specific surface area will adsorb more active intermediate products during the operation and have a larger double-layer capacitance effect, resulting in a greater reverse-current impact during the start- stops of the electrolyzers.
Therefore, in commercial alkaline cells, there are practical operational bottlenecks in increasing the specific surface area of electrodes to enhance activity. But the truth is that the electrodes with a high specific area will inevitably lead to a decrease in stability while bringing high activity.
In the development and application of commercial electrodes of Baoshilai, the safety and stability of electrodes are always given top priority. In electrode research and development, we are always seeking the balance point between performance and the stability of the coating structure, which is of crucial importance for manufacturing commercial electrode products. Therefore, for electrolyzers that are not specifically designed or for non-short-term demonstration projects, Baoshilai has always maintained a cautious attitude towards the BSL-7.0 electrode represented by high-specific-surface-area electrodes and the obligation to inform customers.
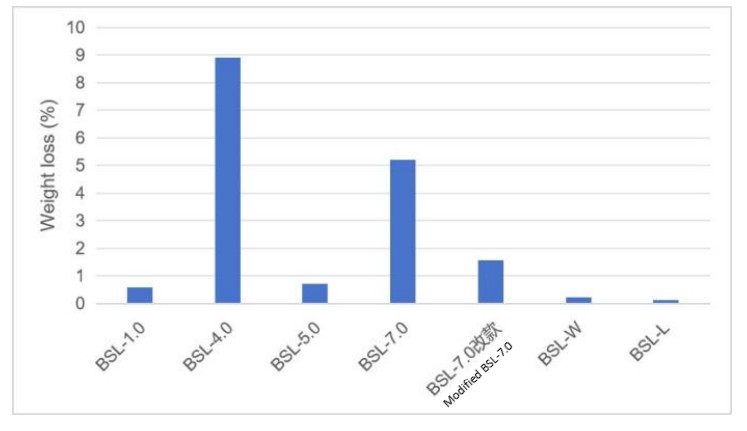
Fig.6 Ultrasonic weight loss ratio (test conditions: power of 300 W, temperature of 60℃, solution of 20 wt% KOH, ultrasonic treatment time of 2 hours).
2. Baoshilai’s Novel Electrodes
The iterative of Raney nickel electrodes based on thermal spraying often requires comprehensive consideration of the catalytic activity, specific surface area, coating bonding strength, and anti-reverse current performance of the electrodes. Raney nickel electrodes made by thermal spraying have undergone practical operation verification for over a hundred years in the West and more than thirty years in China, and have been proven to be applicable in alkaline water electrolyzers for a long time (> 30 years). The collaborative optimization of the specific surface area, porous structure, coating bonding strength, anti-reverse current characteristics, and process parameters of Raney nickel coatings is the key research direction for Raney nickel electrodes in the future. After more than two years of development, the fifth- generation Raney nickel electrode (BSL-5.0) of Baoshilai has been successfully adopted by more than 80% of domestic electrolyzers customers and widely used in large-scale domestic demonstration projects, featuring low energy consumption, high safety, and good product consistency.
There is a contradiction between the performance and stability of typical Raney nickel electrodes. To seek a balance, it is inevitable to sacrifice some performance to meet the requirements for the stable operation of the electrolyzers. In the future, there will surely be a new balance point for Raney nickel electrodes, which can better balance performance and stability. However, under the premise of meeting stability, the improvement of performance will be increasingly limited. Therefore, Baoshilai has researched on new electrode materials and new processes, and has developed many technical routes as technical reserves. Electrodes based on new materials and new processes need to not only have better performance and stability than the BSL-5.0 Raney nickel electrodes but also have outstanding advantages in withstanding frequent start-stop of the electrolyzers, anti-shedding, safe and efficient manufacturing processes, and being environmentally friendly.
The activation of Raney nickel electrodes is usually somewhat polluting and requires a large amount of water. Developing non-activated electrodes, on the one hand, meets the requirements of sustainable development, and on the other hand, it can simplify the electrode preparation and shorten the electrode preparation cycle. In concept, non-activated electrodes should be non-Raney nickel electrodes, that is, they do not contain chemical components soluble in strong alkali, and there is no process of the coating components re-dissolving and forming a porous structure during the electrode operation.
Both the BSL-W series and BSL-L series developed by Baoshilai are non-activated electrodes. By starting the design from the material end and improving the intrinsic activity per site of the coating material, high catalytic activity can be achieved without requiring a large electrode specific surface area. Therefore, this series of coating materials do not need to add sacrificial materials such as aluminum and zinc to prepare porous structures, nor do they need to add soluble elements such as molybdenum, iron, and cobalt to change the active sites.
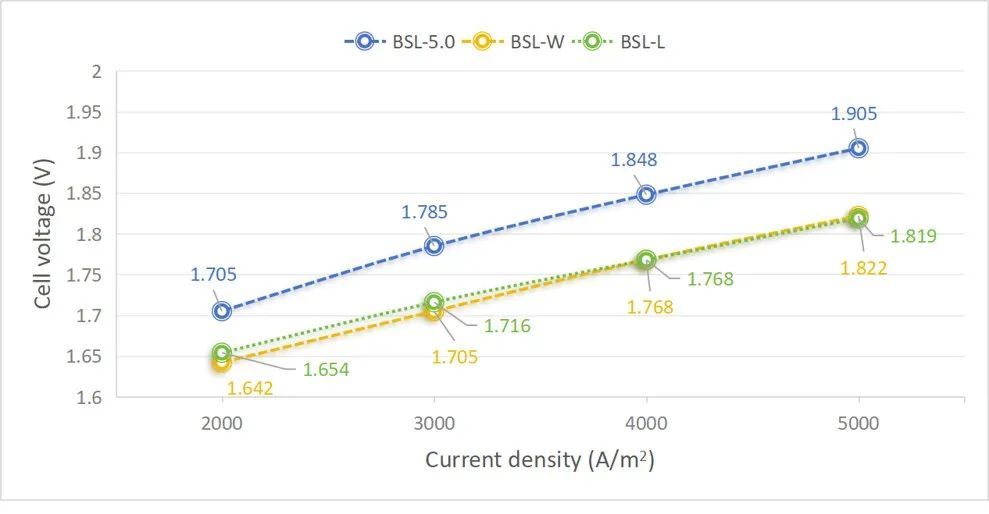
Fig.7 Test performance of electrolyzers (test conditions: 10 cells with plate and mesh structure, electrode diameter of 252 mm, 46 meshes/0.25 mm, PPS diaphragm thickness of 0.8 mm, surface resistance ~0.2 Ω·cm², plain anode mesh, 30 wt% KOH, temperature of 82℃, pressure of 0.2 MPa).
2.1 BSL-W Series Electrodes
The BSL-W series adopts the current commercial thermal spraying process-flame spraying, and has the advantages of simple process, environmental protection, and rapidity. The BSL-W series electrodes have excellent electrocatalytic hydrogen evolution performance. Compared with BSL-5.0, the hydrogen evolution overpotential is reduced by 70-90 mV@5000 A/m², and the cell voltage after cell test is reduced by >70 mV. In addition, the BSL-W series has carried out targeted material modification and process optimization for the problem of reverse current impact existing in the start-stop of the electrolyzers. Compared with BSL-5.0, its reverse current resistance performance is improved by ~50%. The sample can withstand nearly 400 times under the reverse current impact of +10000 A/m² for 30 seconds without performance attenuation and coating shedding. Under the same circumstances, BSL-5.0 can withstand 260 impacts. The excellent reverse current resistance performance endows the BSL-W series with the ability to adapt to fluctuating power sources of wind and solar energy and withstand the frequent start-stop of the electrolyzers.
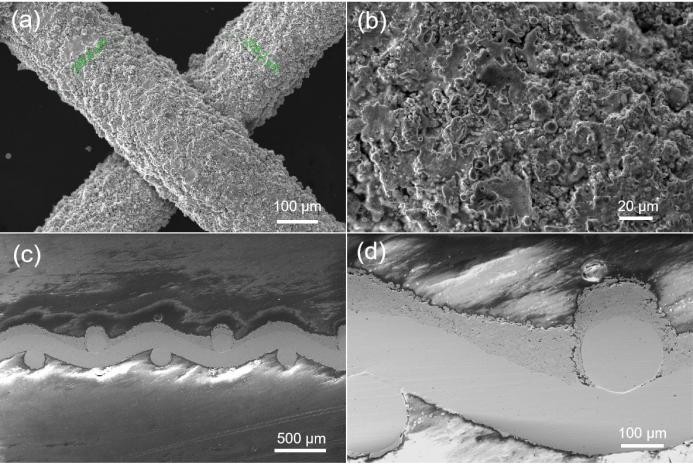
Fig.8 (a-b) Surface SEM images of the BSL-W series, (c-d) cross-sectional SEM images of the BSL-W series.
2.2 BSL-L Electrodes
The BSL-L series electrodes are advanced products of a new process and material system. They adopt a new chemical composite plating process and have a completely dense coating structure. The coating and the nickel mesh substrate are chemically bonded, with the advantages of being dense and high bonding strength. In ultrasonic oscillation, the BSL-L series electrodes can free from any disturbance or strong alkali corrosion. The ultrasonic weight loss rate in strong alkali is two orders of magnitude lower than that of electrodes made by thermal spraying. As a reserve electrode product newly developed by Baoshilai, BSL-L completely abandons the technical route of improving electrode performance by increasing the specific surface area. Instead, it adopts a highly active coating material. Under the condition that the specific surface area is the same as that of a plain nickel mesh, the cell voltage of the electrode can be reduced by 60-100 mV@5000 A/m² compared to BSL-5.0.
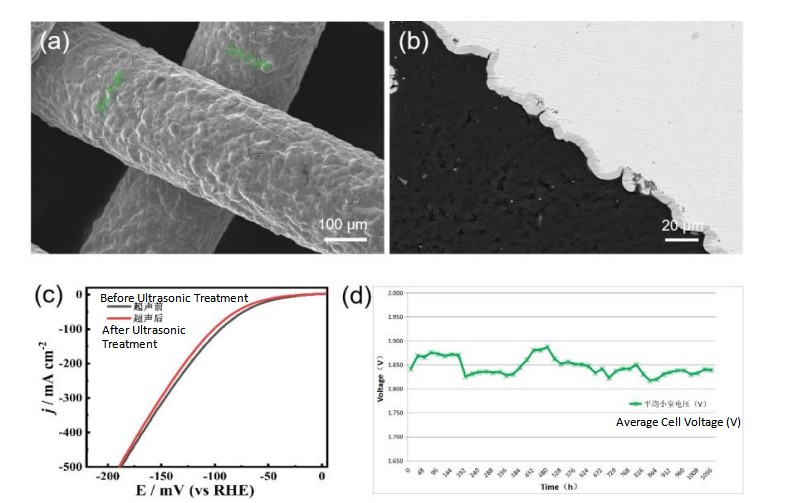
Fig.9 (a) Surface SEM images of the BSL - L series, (b) cross-sectional SEM images of the BSL - L series, (c) performance of the BSL-L series before and after ultrasonic treatment, (d) stability test of the BSL - L electrolyzer.
3. Prospects
As electrolysis of water is the most primary means of green hydrogen production, the production and manufacturing of its core components should be sustainable. On the basis of developing highly active electrodes, Baoshilai will also continuously pursue environmental-friendly development,save resources, and promote the harmonious co-existence of humans and nature, continuously improving the technical content and market competitiveness of products, and serving the sustainable development of the hydrogen energy industry.
Awesome! Share to:
Related Posts
- Baoshilai New Material: Honored with 2nd Prize in China Surface Eng. Tech Progress
- Riding the Wave of Success with Orders from Domestic and Overseas Market
- Baoshilai New Materials: Shining in Jiangsu's New Energy Field and Winning Honors
- Academician Javad Mostaghimi from University of Toronto Visits Baoshilai to Explore the Future of Advanced Coating Technologies Together
- Veterans of "Three Established Water Electrolysis Companies" Gather at Baoshilai for Guidance and Common Development

First class quality service and professional after-sales team.
Get in Touch
*We respect your confidentiality and all information are protected.
